INTRODUCTION- The perfectly regular crystal structures that have been considered up to now are called ideals crystal in which atoms were arranged in a regular way. An ideal crystal can be described in terms a three-dimension ally periodic arrangement of points called lattice and an atom or group of atoms associated with each lattice point called motif:
However, there can be deviations from this idealistic.
These deviations are known as crystal imperfection. These imperfections affect the properties of crystal, such as mechanical strength, chemical reaction, electrical properties, etc. to a great extent.
TYPES OF IMPERFECTION:-
1. Point Imperfections
2. Line Imperfections
3. Surface And Grain Boundary Imperfections
Defects
|
Dimensional
|
Examples
|
Point defects
|
0
|
Vacancy
|
Line defects
|
1
|
Dislocation
|
Surface defects
|
2
|
Free surface, Grain boundary
|
(1):- POINT IMPERFECTIONS: - In some cases, when atoms missing or in irregular places in the lattice (vacancies, interstitial, substitution).These imperfections are always present in crystals and their presence results in a decrease in the free energy. The number of defects at equilibrium concentration at a certain temperature can be compound as;
n = Ne-Ed/kT
n = Number of imperfections
N = Number of atomic sites per mole
K = Boltzmann’s constant
Ed = the free energy required to from the defect
T = Absolute temperature
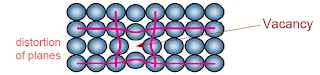
(a)- Vacancies- A lattice position that is vacant because the atom is missing. It defect may arise due to increased thermal energy causing individual atoms to jump out of their position of lowest energy. The thermal vibrations of atoms increase at high temperatures. The vacancies may be single or two or more may condense into a di-vacancy or tri-vacancy. Vacancies exist in a certain proportion in a crystal at thermal equilibrium, leading to an increase in randomness of the structure.
Where Ns is the number of regular lattice sites, kB is the Boltzmann constant, Qv is the energy needed to form a vacant lattice site in a perfect crystal, and T the temperature in Kelvin (note, not in oC or oF).
Using this equation we can estimate that at room temperature in copper there is one vacancy per 1015 lattice atoms, whereas at high temperature, just below the melting point there is one vacancy for every 10,000 atoms.
(b)- Interstitial - An atom that occupies a place outside the normal lattice position. It may be the same type of atom as the others (self interstitial) or an impurity interstitial atom.
(c)-Substitutional Impurity- It may be produce compositional defects in the crystal structure. When impurity in the form of the foreign atoms occupies lattice sites where regular atoms are missing, they produce substitutional impurity.
(d)- Frankel Defect- A frankel defect is closely related to interstices. An ion displaced from the lattice site into an interstitial site is called a Frankel Defect. Closed packed structures have fewer intestacies & Frankel Defects because additional energy is required to force the atom into a new position.
(e)-Schottky Defect- This is closely related to vacancies and is obtained when an atom or ion is removed from a normal lattice site and replaced by an ion on the surface of the crystal. Both vacancies and Schottky Defects facilitate atomic diffusion.
(2):- LINE IMPERFECTIONS / DISLOCATIONS: - A linear
Disturbance of an atomic arrangement, which can very easily occur on the slip plane through the crystal, is known as line dislocation. It is two dimensional line defects & it may also be concluded that be is the region of localized lattice disturbance separating the slipped and unslipped regions of a crystal.
These are formed in the process of solidification of metal and mainly in their plastic deformation of strain hardening. Yield point, creep and fatigue and brittle fracture.
Causes of dislocation are:
1. thermal stress or external stresses causing plastic flow
2. crystal growth
3. phase transformation
4. Segregation of solute atoms causing mismatches.
There are two types of dislocation:
(a) Edge Dislocations
(b) Screw dislocations
(a) Edge Dislocations:- An edge dislocation formed by adding an extra partial plane of atoms to the crystal. The poison of the dislocation line is marked by the symbols ┴ and ┬ indicating the involvement of extra planes from the top (positive sign) and bottom (negative sign) of the crystal, respectively. The vertical line of the symbol ┴ points in the direction of the dislocation line in the extra partial plane. The dislocation line is a region of high energy than the rest of the crystal. The lattice above the dislocation line is in a state of compression, whereas below this line, the lattice in tension.
To describe the size and the direction of the main lattice distortion caused by a dislocation we should introduce so called Burgers vector b. To find the Burgers vector, we should make a circuit from atom to atom counting the same number of atomic distances in all directions. If the circuit encloses a dislocation it will not close. The vector that closes the loop is the Burgers vector b. Dislocations shown above have Burgers vector directed Perpendicular to the dislocation line.
(a) Screw Dislocations:- the formation of a screw dislocation
by a perfect crystal and a plane cutting part way through it
are also shown. The geometry of the screw dislocation has an interesting effect on the solidification process
A Screw dislocation has its displacement or Burger vector
Parallel to the linear defect but there is a distortion of the plane. In this the atoms are displaced in two separate plane perpendicular to each other and the distortion follows a helical or screw path, both right hand and left hand screw are possible. In these types of dislocation, shear stresses are associated with adjacent atom and extra energy is involved along the dislocation. A screw dislocation does not exhibit climb motion.
1. The force required to form and move a screw dislocation probably somewhat greater than that required to initiate an edge dislocation.
2. Plastic deformation is possible under low stress, without breaking the continuity of the lattice.
3. Screw dislocation causes distortion of the lattice for considerable distance from the center of the line and takes the from of spiral distortion of the planes. Dislocation of both types (combinations of edge and screw) are closely associated with crystallization as well as deformation.
Dislocation of both types (Mixed/partial dislocations)
In general, there can be any angle between the Burgers vector b (magnitude and the direction of slip) and the line vector t (unit vector tangent to the dislocation line)
b ^t ÞEdge dislocation b ççt ÞScrew dislocation
(3) SURFACE IMPERFECTIONS: - these defect are two –dimensional and due to a change in the stacking of atomic planes on or across a boundary. Such imperfection may include external & internal defects (grain boundary, tilt boundary, twin boundary etc.)
External free surface: - the external surface of the material is an imperfection itself because the atomic bonds do not extend beyond it since these surface atom are not entirely surrounded by other, they posses higher energy than internal atoms. Surface atoms have neighbors on only one side while atoms inside the crystal have neighbors on both sides.
Internal Surface:-
(a) Grain Boundary:- Polycrystalline material comprised of many small crystals or grains. The grains have different crystallographic orientation. There exist atomic mismatch within the regions where grains meet. These regions are called grain
Boundaries.
Surfaces and interfaces are reactive and impurities tend to
Segregate there. Since energy is associated with interfaces, grains tend to grow in size at the expense of smaller grains to minimize energy. This occurs by diffusion, which is accelerated at high temperatures. |
| Add caption |
(b) Tilt Boundaries: - Low angle grain boundary is an array of aligned edge dislocations. This type of grain boundary is called Tilt Boundary (consider joint of two wedges)
Transmission electron microscope image of a small angle tilt boundary in Si. The red lines mark the edge dislocations; the blue lines indicate the tilt angle.
(c) Twin Boundary: - Space imperfections which separate two like orientations and look like mirror image of each other are called Twin Boundary.
Low-energy twin boundaries with mirrored atomic positions across boundary may be produced by deformation of materials. This gives rise to shape memory metals, which can recover their original shape if heated to a high temperature. Shape-memory alloys are twinned and when deformed they untwine. At high temperature the alloy returns back to the original twin configuration and restore the original shape.
(d) Stacking faults: - This type of imperfection may arias where there is only a small dissimilarity between the stacking sequences of close packed planes in FCC and HCP. It is possible for one atom layer to be out of sequence relative to the atom of the layer above and below, giving a fault.
For example, the stacking sequence of an ideal FCC crystal may be described as ABC ABC.... but the stacking fault may change the sequence to ABC ACAB.
REFRENCE
· Material science by G.K. Narula,V.K.Gupta,
· Lecture 5 of Rick Holt, Queen’s University , Canada,
· From internet,
· From classnotes,
THE END